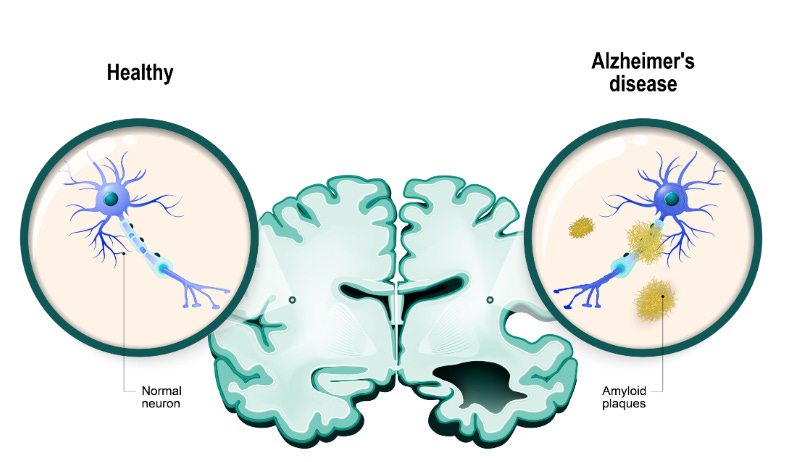
Alzheimer’s disease is a neurodegenerative disorder that affects about 55 million people worldwide. It’s characterized by memory loss, cognitive decline, and behavioral changes that seriously affect daily life. While the exact causes of Alzheimer’s are unknown, many existing theories point to protein dysfunction which results in decreased neural connections and neuron death.

Since the exact cause of Alzheimer’s is still unknown, current treatments focus on alleviating symptoms. These treatments cannot stop or reverse the progression of the disease, highlighting the urgent need for further research and new therapeutic approaches.
One interesting area of exploration for Alzheimer’s treatment lies in psychedelics. Psychedelics are known to have a significant role in promoting neurogenesis (the growth of new neurons), synaptogenesis (the formation of new synaptic connections), and overall neuroplasticity. Theoretically, psychedelics could counteract some of the neurodegenerative mechanisms at play in Alzheimer’s. Additionally, there are a few other mechanisms by which psychedelics may alleviate dysfunction commonly seen in Alzheimer’s.
This article will explore how psychedelics may address key pathological features of Alzheimer’s. It will explore the following factors:
Amyloid Beta Plaques
Neurofibrillary Tangles
Neurotransmitter Dysfunction
Excitotoxicity
Neuroinflammation
BDNF and Neuroplasticity
At the end of the article, I will discuss the unique and pressing ethical challenges and implications involved in using psychedelics with an Alzheimer’s patient population.
1. Amyloid Beta Plaques
Role in Alzheimer’s
In a healthy brain, amyloid proteins play a role in synaptic plasticity, neuroprotection, and neural development. In Alzheimer’s, amyloid beta accumulates due to either overproduction or impaired clearance from the brain. This results in the formation of amyloid beta plaques, which are extracellular clumps of amyloid beta protein. These plaques are often found in areas of the brain responsible for memory and emotional behavior, like the hippocampus, basal forebrain, and amygdala. These plaques disrupt neuronal communication and ultimately lead to cell death. Interestingly, it’s not clear if the plaques are side effects of Alzheimer’s, or if they cause symptoms of Alzheimer’s.
Existing Treatments
One treatment that targets amyloid beta plaques is aducanumab, an anti-amyloid beta antibody which targets and removes plaque buildup. However, it has been surrounded by controversy; an FDA review found that data did not support the efficacy of high-dose aducanumab in Alzheimer’s patients. Ultimately, in November 2024, Biogen, its manufacturer, discontinued aducanumab, citing reasons unrelated to efficacy.
Psychedelic Interactions
Researchers found that the classic psychedelic N,N-dimethyltryptamine, more commonly known as DMT, has the potential to be a therapeutic treatment for Alzheimer’s. In one study, they found promising results in a mouse model, where DMT lowered amyloid beta plaque in the hippocampus and prefrontal cortex, and also alleviated cognitive impairment. DMT’s neuroprotective effects may be linked to its activation of Sig-1r, which is a receptor that plays a key role in cellular stress response. Researchers concluded that DMT has potential to be a preventative and therapeutic treatment for Alzheimer’s.
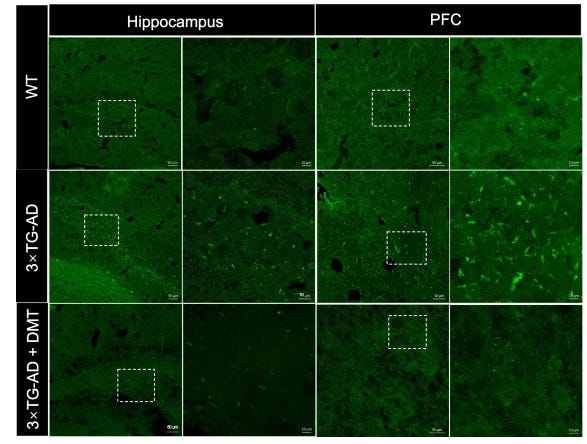
2. Neurofibrillary Tangles (NFTs)
Role in Alzheimer’s
The microtubule-associated protein tau is normally involved in helping maintain neuronal shape and structure. In Alzheimer’s, abnormal hyperphosphorylation causes tau to clump together and form tangles referred to as NFTs. These NFTs disrupt tau’s normal role of stabilizing the microtubules. Functionally, NFTs impair communication between neurons, causing neurons to die. Similar to amyloid beta plaques, it’s not clear if NFTs are side effects of Alzheimer’s or if they cause symptoms of Alzheimer’s.

Existing Treatments
Compared to the number of treatments targeting amyloid beta plaques, there have been fewer drugs designed to target NFTs in Alzheimer’s. Those that do exist, such as tau aggregation inhibitors (like the methylene blue derivative LMTX) or kinase inhibitors (like the glycogen synthase kinase-3 (GSK-3) inhibitor tideglusib), have shown limited success and remain less well-known due to mixed or inconclusive trial results. While these therapies aim to prevent tau aggregation or reduce harmful hyperphosphorylation, none have proven to be effective in large-scale clinical studies.
Psychedelic Interactions
A recent study found that the classic psychedelic psilocybin reduced levels of phosphorylated tau in a rat model of traumatic brain injury (TBI). While TBI is not a neurodegenerative disease, it shares similar pathology with Alzheimer’s and other neurodegenerative disorders, including the hyperphosphorylation and aggregation of tau.
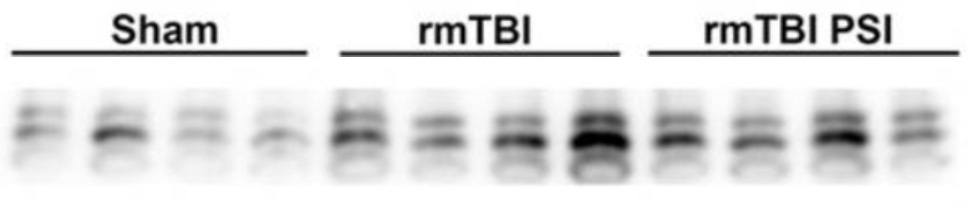
This reveals that psilocybin has the potential to actively target a hallmark pathological feature of Alzheimer’s. This finding is promising for Alzheimer’s treatment, but more broadly it highlights a novel mechanism by which psilocybin (best known for its effects on 5-HT2A receptors) can impact the brain, potentially addressing tau-related pathology in a way that traditional treatments have yet to achieve. It’s exciting to see how psychedelics like psilocybin may offer unexpected therapeutic benefits beyond their more commonly understood roles in serotonin modulation.
3. Neurotransmitter Dysfunction
Neurotransmitters are chemicals that allow neurons to communicate with each other, transmitting signals across synapses in the brain. Two neurotransmitters of notable interest in Alzheimer’s pathology are acetylcholine (ACh) and serotonin (5-HT).
Acetylcholine: Role in Alzheimer's
ACh is a neurotransmitter essential for learning, memory, and cognitive function. In Alzheimer’s, ACh levels are significantly reduced due to the degeneration of cholinergic neurons in the brain.
Acetylcholine: Existing Treatments
Current treatments for Alzheimer’s targeting ACh deficits rely on acetylcholinesterase inhibitors, which slow the breakdown of ACh and temporarily improve cognition and memory loss. However, there is no evidence that acetylcholinesterase inhibitors improve behavioral deficits seen in Alzheimer’s. Moreover, these treatments are limited, as they do not halt disease progression or repair underlying neuronal damage.

Acetylcholine: Psychedelic Interactions
The effect of psychedelics on the ACh system remains unexplored; research has not really examined how psychedelics modulate the ACh system.
Serotonin: Role in Alzheimer’s
Classic psychedelics primarily influence the serotonin system, particularly the 5-HT2A receptor. This is especially relevant, as serotonin plays a key role in both the mood and cognitive decline associated with Alzheimer’s. Lower serotonin levels in Alzheimer’s patients have been linked to accelerated cognitive decline and lowered memory performance. Specifically, studies have found that decreased 5-HT2A receptors in the temporal cortex correlates with a faster rate of cognitive decline in Alzheimer’s.
Serotonin: Existing Treatments
Selective serotonin reuptake inhibitors (SSRIs), commonly used to treat depression, have shown mixed results in the context of Alzheimer’s. On one hand, SSRI use has been associated with protective effects, such as lowering the risk of developing Alzheimer’s, improving brain function in regions affected by the disease, and even reducing biological markers like phosphorylated tau. Long-term SSRI use has also been linked to restored neuronal activity in the dorsal raphe nucleus, a key brain area impacted by Alzheimer’s.

However, the data is not entirely consistent. Some studies suggest that SSRI use may accelerate cognitive decline in dementia patients. This contradictory evidence highlights the complexity of the serotonin system in Alzheimer’s and highlights the need for further research.
Serotonin: Psychedelic Interactions
Classic psychedelics like psilocybin and LSD offer a unique avenue for investigation, particularly for their ability to modulate serotonin signaling through 5-HT2A receptors. Many studies have indicated psychedelics’ promise in treating treatment-resistant depression (TRD), a condition where traditional antidepressants like SSRIs fail, raising the question of whether they could also address some of the cognitive and mood symptoms associated with Alzheimer’s.
While there is strong evidence supporting the effectiveness of psychedelics in managing depression, there are currently no direct studies investigating their potential to correct serotonin deficits in Alzheimer’s models. However, given the overlap in serotonin-related mechanisms between TRD and Alzheimer’s-related depression, it’s reasonable to hypothesize that psychedelics might offer similar benefits in the context of Alzheimer’s.
The success of psychedelics in treating depression may indicate their potential to alleviate the depressive and cognitive symptoms linked to Alzheimer’s. Further research is needed to fully understand this connection, but the potential is definitely promising.
4. Excitotoxicity
Role in Alzheimer’s
Excitotoxicity, caused by overactivation of the glutamate receptor NMDAR (N-methyl-D-aspartate receptor), contributes to neuronal death in Alzheimer’s. Astrocytes, which are support cells in the brain that help maintain neuronal health by clearing excess neurotransmitters, fail to remove surplus glutamate in Alzheimer’s, leading to overstimulation of NMDARs and triggering reactive oxygen species (ROS)-related cell death.
Existing Treatments
Memantine, an NMDAR antagonist, is a commonly prescribed treatment for Alzheimer’s. By blocking NMDAR overactivity, memantine prevents excitotoxicity, protecting neurons from cell death. Memantine has been shown to slow cognitive decline in a clinical trial of Alzheimer’s patients. However, notably, memantine does not address the other pathological features of Alzheimer’s discussed in this article, so it is often used in combination with other therapeutics.
Psychedelic Interactions
Ketamine, an NMDAR antagonist, has shown potential as a treatment by reducing excitotoxicity and offering neuroprotective effects. Ketamine may act via a similar mechanism as memantine, in that it could prevent overactivity in NMDARs.

However, its impact is complex, as research suggests it can be either neuroprotective or neurotoxic depending on the context. Moreover, other studies suggest that ketamine increases ROs-related cell death, as it results in an increase in NMDAR expression. These findings emphasize the need for further research.
5. Neuroinflammation
Role in Alzheimer’s
Chronic neuroinflammation is another hallmark of Alzheimer’s pathology, exacerbating neuronal damage and accelerating disease progression. In patients with Alzheimer’s, there is an increase in inflammatory markers, including elevated cytokine levels and heightened activation of immune cells. Additionally, microglial cells, which normally help clear debris and protect neurons, often become dysfunctional in Alzheimer’s, further contributing to heightened neuroinflammation.
This persistent inflammation damages neurons, and also disrupts synaptic function, worsening the cognitive decline and memory loss associated with Alzheimer’s.
Existing Treatments
Current treatments aimed at addressing neuroinflammation in Alzheimer’s primarily focus on restoring the functionality of microglia and astrocytes. Nonsteroidal anti-inflammatory drugs (NSAIDs), which are commonly used for their anti-inflammatory effects, have been investigated for their potential to reduce the risk of Alzheimer’s. However, the evidence remains controversial, with some studies suggesting benefits while others fail to show significant effects.
One notable study examined the potential of naproxen, an NSAID, in treating Alzheimer’s symptoms. This study had to be halted due to an increased risk of cardiac disease in participants. Current treatment guidelines do not recommend NSAIDs for the prevention or treatment of dementia or cognitive impairment, citing insufficient evidence of efficacy and potential risks associated with their use. This highlights the need for safer and more effective therapies targeting neuroinflammation in Alzheimer’s.
Psychedelic Interactions
Some classic psychedelics, particularly psilocybin and LSD, have shown promising anti-inflammatory effects via their action on 5-HT2A receptors, which could potentially assist in mitigating the chronic inflammation linked to Alzheimer’s.
Studies have shown that psilocybin can decrease the levels of inflammatory cytokines, such as TNF-α and IL-6, which are often elevated in Alzheimer’s. Additionally, psilocybin has been observed to modulate microglial activity, inhibiting their phagocytic activity and shifting them from a pro-inflammatory state to a more neuroprotective, anti-inflammatory phenotype. This underscores the potential of psychedelics as therapeutic agents for managing neuroinflammation in Alzheimer’s.
6. BDNF and Neuroplasticity
Role in Alzheimer’s
One of the most commonly understood deficits in Alzheimer’s is the decrease in brain-derived neurotrophic factor (BDNF) and impaired neuroplasticity. BDNF plays a crucial role in supporting the survival, growth, and maintenance of neurons, while neuroplasticity is essential for learning, memory, and adaptation. In Alzheimer’s, reduced BDNF levels and impaired plasticity contribute to cognitive decline and neuronal dysfunction.
Existing Treatments
One current treatment aimed at targeting impaired BDNF levels in Alzheimer’s is cerebrolysin. Cerebrolysin was found to increase BDNF in Alzheimer’s patients, as it upregulates the gene responsible for BDNF expression. Moreover, clinical studies suggest that it may improve cognitive function in Alzheimer’s patients, particularly in those carrying the ApoE4 allele, a genetic risk factor for Alzheimer’s.
Although these treatments provide benefits, their effects do not fully address the extensive plasticity deficits seen in Alzheimer’s, emphasizing the need for further research into innovative therapies that directly target BDNF and plasticity pathways.
Psychedelic Interactions
Psychedelics have been shown to promote BDNF expression and enhance neuroplasticity across numerous studies. Psychedelics are classified as being psychoplastogens, defined by their ability to rapidly produce neural plasticity, supporting both neurogenesis and synaptogenesis.

Atypical psychedelics like ketamine have been shown to increase BDNF levels, highlighting the potential for psychedelics to counteract plasticity and BDNF deficits associated with Alzheimer’s.

Interestingly, in Alzheimer’s mouse models, KOR agonists have shown promise in enhancing neuroplasticity. This indicates that other kappa opioid receptor (KOR) agonists, such as salvia, may play a similar role in improving plasticity. Research around salvia is much less extensive than other psychedelics such as LSD or psilocybin, so further research is necessary for a more comprehensive understanding.
Ethical Considerations
There are many challenges with administering psychedelics to an older, cognitively impaired, and potentially more vulnerable population. Potential risks include increased anxiety, worsened cognitive deficits, and difficulty obtaining informed consent, due to the nature of conditions like Alzheimer’s.
To address these concerns, there is a critical need for carefully designed clinical trials. Such studies must prioritize patient safety while rigorously evaluating the efficacy of psychedelics in alleviating symptoms of Alzheimer’s, ensuring that any potential benefits outweigh the risks.
As summarized in a paper published in the American Journal of Geriatric Psychiatry:
“Ultimately, the path to using psychedelics as therapeutic adjuncts in dementia care is daunting, but worth consideration when drug development is so fraught with challenges. Although we acknowledge legitimate discomfort many may feel toward using psychedelics… there is also wisdom in the insight commonly attributed to Albert Einstein that “if at first the idea is not absurd, then there is no hope for it.”” (Quote source)
Conclusion
Psychedelics show promising potential to target multiple key aspects of Alzheimer’s pathology, including reducing amyloid beta plaques, decreasing neurofibrillary tangles, modulating serotonin dysfunction, protecting against excitotoxicity, reducing chronic neuroinflammation, and restoring deficits in neuroplasticity and BDNF levels.
Taken alongside the limited success of current treatments, these findings indicate that it may be worth further researching the potential of psychedelics as treatment for Alzheimer’s. That being said, it is crucial to approach this with a realistic approach, avoiding getting swept up in the hype that often surrounds psychedelic therapy. Given the unique vulnerability of Alzheimer’s patients, it is especially necessary to emphasize rigorous, ethical research to truly understand the role that psychedelics could have in the future of Alzheimer’s treatments.
At present, there is a lack of research directly investigating psychedelics in Alzheimer’s disease. Most existing studies have focused on animal models or on how psychedelics interact with individual pathological features of Alzheimer’s, rather than examining their effects in rigorous clinical trials in human patients. Given how widespread and devastating Alzheimer’s is, this research gap feels especially urgent.
In my view, there’s enormous potential here that we’re only beginning to scratch the surface of. For instance, what if psychedelics could be used not just to treat symptoms but could be used prophylactically to slow disease progression before extensive neurodegeneration occurs? Or what if microdosing regimens could provide a safer alternative, aiming to make use of the neuroplastic benefits without intense perceptual/subjective effects? These are directions that I think have yet to be fully explored, and they could open new possibilities for how we approach such a complex and challenging disease.
Still, to reiterate, it’s essential to balance this excitement with caution and realism. Especially in recent years, psychedelics have attracted significant media attention. Without a rigorous scientific approach, the potential of psychedelics is at risk due to this sensationalism.


Love this! Great work.